By Caitlin Rothermel
Have you heard about the “bivalent” and “monovalent” COVID vaccines? These are the old and new versions of the Pfizer and Moderna injections. The original injection is monovalent because it contains messenger RNA (mRNA) for only the Wuhan strain of SARS-CoV-2. The bivalent booster has mRNA from the Wuhan strain and the Omicron strain variants BA. 4 and 5.1
There has been some confusion as to whether enough clinical research was done to evaluate the final bivalent injection. Was product approval really only based on data from eight mice?
I did the research, and I have some answers. Unless noted otherwise, all my information is from the U.S. Centers for Disease Control (CDC), the U.S. Food and Drug Administration (FDA), or is published in peer-reviewed medical journals. The references are online if you read this article at vashonloop.com.
So, was the mRNA bivalent booster approved based on mouse data only? No – it was also based on information from nearly 3,000 injected humans, aged 18 to 55 years, who received a bivalent injection.2,3 So, what’s with the mouse story, then? Well, the bivalent injection received by those humans was not the same as the bivalent injection now on the market – only the mice received that.
First, the human studies. Per data presented to the FDA in June 2022, the monovalent injection was compared to a bivalent injection that contained mRNA for the Wuhan strain and Omicron variant BA. 1. Note that BA. 1 is different than the variants (4/5) targeted by the final booster. The studies compared the safety of different injection regimens (all patients received some sort of active injection) and looked at patient’s neutralizing antibody response.2-4 The role of neutralizing antibodies is to defend cells from infectious particles by deactivating their biologic effect.
Neither study looked at how effective the injection was in preventing infection. Primarily, the study investigators wanted to know whether the bivalent injection triggered a neutralizing antibody response to Omicron that was greater than the neutralizing response achieved using the monovalent injection. And it did – the study goal was reached.2-4
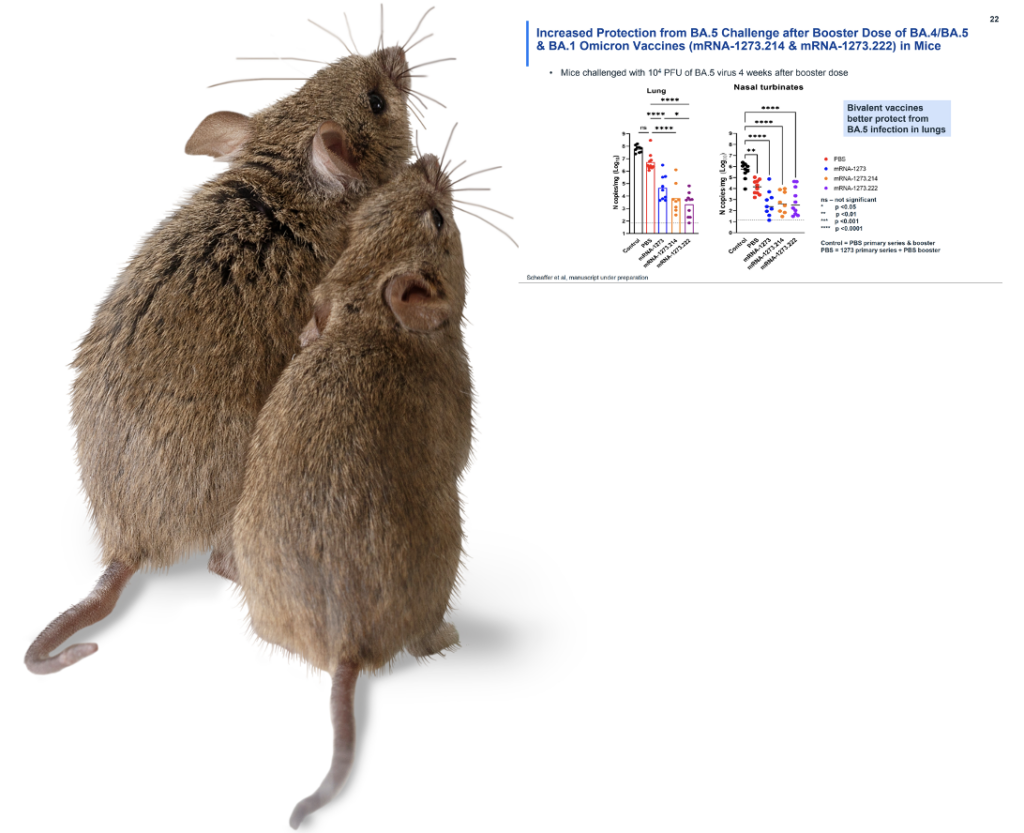
Next, the mice. For Pfizer, the final bivalent injections were tested on 8 mice, and Moderna conducted tests on 10 mice.4,5 The mice were evaluated for neutralizing antibodies, and again, the bivalent injection did its job: In the Pfizer data, after 104 days, the BA. 4/5 antibody count for each mouse reached a pre-set level of detection.4 It has been subsequently noted by sharp-eyed online observers that this antibody count varied tremendously by mouse, with concentrations that ranged from 300 to 22,000 per part. This result raises as-yet unanswered questions – what kind of antibody distribution we will see in humans, and what will that mean?4,6 In September 2022, we learned that when the mice in the Moderna study were exposed to BA. 5, they developed COVID (based on viral counts in the lung and nose going beyond the limit of detection).5
The use of mice has been defended as being a common study approach used in other situations, like influenza vaccine development. This is true. But, according to European influenza vaccine development guidelines, a multiplicity of mouse (and other animal) studies must be conducted to look at how the drug travels through the body, what side effects it causes, and how it affects a number of immune system functions.7
At this point, it is completely reasonable to be skeptical as to whether more boosting is the best solution to our current predicament. In the past month alone, mRNA from the SARS-CoV-2 injections has been found in human breastmilk8 and in the liver cells of a patient with hepatitis;9 an autopsy study has also isolated spike protein from the injection in the heart and brain.10 We were initially told the injection would stay localized to the arm, within the thick deltoid muscle that surrounds the injection site.11 This is clearly not the case for all people, and the more times you inject, the more times you take this risk.
Recently, national research – conducted in countries where it is possible to study the full population – shows diminishing benefits with each injection, and strong protection from reinfection in patients with natural immunity.12-14 Most of us have natural immunity. As of February 2022, 58% of U.S. adults had serum antibody evidence of a previous SARS-CoV-2 infection, as did 75% of children and adolescents.15
In terms of the national data, in Israel, a retrospective (ie, backwards-looking) evaluation of more than 389,000 people showed that protection from COVID infection decreased notably within three months of receiving the injection – from 53% to 17%.12 Similarly, a national study of Qatari patients found, “Rapidly waning vaccine protection after the second and third doses, but slowly waning protection from previous infection.”13 Most recently, a study in Iceland identified and compared all the residents of this island nation who had a SARS-CoV-2 infection between December 2021 and February 2022 – about 11,500 people. Investigators found that, “The probability of reinfection increased with time from initial infection…and was higher among persons who had received 2 or more doses compared with 1 dose or less of vaccine.”14
As I write, there is quiet, but growing, international pullback from the injection program and an increasing recognition that what was once an emergency now requires reconsideration. The United Kingdom no longer recommends the mRNA injections for children under 12 years,16 Sweden no longer recommends them for most children aged 12-17 years,17 and Denmark no longer provides injections to people younger than 50. Their rationale? “The purpose of vaccination is not to prevent infection with COVID-19, and people aged under 50 are therefore currently not being offered booster vaccination.”18
References
1. U.S. Food and Drug Administration (FDA). Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose [press release]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use. Published August 31, 2022. Accessed October 3, 2022.
2. Hoge S. mRNA-1273.214—Moderna COVID-19 investigational bivalent vaccine (original + Omicron). U.S. Food and Drug Administration (FDA). https://www.fda.gov/media/159492/download. Published June 28, 2022. Accessed October 3, 2022.
3. Chalkias S, Harper C, Vrbicky K, et al. A bivalent Omicron-containing booster vaccine against Covid-19. N Engl J Med. 2022. doi:10.1056/NEJMoa2208343
4. Swanson KA. Pfizer/BioNTech COVID-19 Omicron-modified vaccine options. U.S. Food and Drug Administration (FDA). https://www.fda.gov/media/159496/download. Published June 28, 2022. Accessed October 3, 2022.
5. Miller J. Booster doses of Moderna COVID-19 vaccines in adults, adolescents & children. Presented at: Advisory Committee on Immunization Practices; September 1-2, 2022; Atlanta, GA. https://stacks.cdc.gov/view/cdc/120825.
6. Chudov I. Bivalent booster’s “8-Mice Trial” actually failed. https://igorchudov.substack.com/p/ba5-boosters-8-mice-trial-actually. Published August 31, 2022. Accessed October 3, 2022.
7. European Medicines Agency. Guideline on influenza vaccines. https://www.ema.europa.eu/en/documents/scientific-guideline/influenza-vaccines-non-clinical-clinical-module_en.pdf. Published July 21, 2016. Accessed October 3, 2022.
8. Hanna N, Heffes-Doon A, Lin X, et al. Detection of messenger RNA COVID-19 vaccines in human breast milk. JAMA Pediatr. 2022. doi:10.1001/jamapediatrics.2022.3581
9. Martin-Navarro L, de Andrea C, Sangro B, Argemi J. In situ detection of vaccine mRNA in the cytoplasm of hepatocytes during COVID19 vaccine-related hepatitis. J Hepatol. 2022. doi:10.1016/j.jhep.2022.08.039
10. Mörz M. A case report: multifocal necrotizing encephalitis and myocarditis after BNT162b2 mRNA vaccination against COVID-19. Vaccines. 2022;10(10). doi:10.3390/vaccines10101651.
11. Merchant H. Inadvertent injection of COVID-19 vaccine into deltoid muscle vasculature may result in vaccine distribution to distance tissues and consequent adverse reactions. Postgrad Med J. 2022;98(1161):e5. doi:10.1136/postgradmedj-2021-141119
12. Patalon T, Saciuk Y, Peretz A, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13(1):3203. doi:10.1038/s41467-022-30884-6
13. Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387(1):21-34. doi:10.1056/NEJMoa2203965
14. Eythorsson E, Runolfsdottir HL, Ingvarsson RF, Sigurdsson MI, Palsson R. Rate of SARS-CoV-2 reinfection during an Omicron wave in Iceland. JAMA Netw Open. 2022;5(8):e2225320. doi:10.1001/jamanetworkopen.2022.25320
15. Clarke KEN, Jones JM, Deng Y, et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies – United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(17):606-608. doi:10.15585/mmwr.mm7117e3
16. UK Health Security Agency. COVID-19: the green book, chapter 14a. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1102459/Greenbook-chapter-14a-4September22.pdf. Published November 27, 2020. Updated September 5, 2022. Accessed October 3, 2022.
17. Public Health Agency of Sweden. Recommendation on universal vaccination against Covid-19 for children 12-17 years removed. https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2022/september/rekommendation-om-allman-vaccination-mot-covid-19-for-barn-1217-ar-tas-bort/. Published September 30, 2022. Accessed October 3, 2022.
18. Danish Health Authority. Vaccination against Covid-19. https://www.sst.dk/en/English/Corona-eng/Vaccination-against-COVID-19. Updated September 13, 2022. Accessed October 3, 2022.